|
 |
 |
 |
 |
|
|
|






Modifiers of Biotransformation

The relative effectiveness of biotransformation depends on several factors, including species, age, gender, genetic variability, nutrition, disease, exposure to other chemicals that can inhibit or induce enzymes, and dose levels. Differences in species capability to biotransform specific chemicals are well known. Such differences are normally the basis for selective toxicity, used to develop chemicals effective as pesticides but relatively safe in humans. For example, malathion in mammals is biotransformed by hydrolysis to relatively safe metabolites, but in insects, it is oxidized to malaoxon, which is lethal to insects.
Safety testing of pharmaceuticals, environmental and occupational substances is conducted with laboratory animals. Often differences between animal and human biotransformation are not known at the time of initial laboratory testing since information is lacking in humans. Humans have a higher capacity for glutamine conjugation than laboratory rodents. Otherwise, the types of enzymes and biotransforming reactions are basically comparable. For this reason, determination of biotransformation of drugs and other chemicals using laboratory animals is an accepted procedure in safety testing.
Age may affect the efficiency of biotransformation. In general, human fetuses and neonates (newborns) have limited abilities for xenobiotic biotransformations. This is due to inherent deficiencies in many, but not all, of the enzymes responsible for catalyzing Phase I and Phase II biotransformations. While the capacity for biotransformation fluctuates with age in adolescents, by early adulthood the enzyme activities have essentially stabilized. Biotransformation capability is also decreased in the aged. Gender may influence the efficiency of biotransformation for specific xenobiotics. This is usually limited to hormone-related differences in the oxidizing cytochrome P-450 enzymes.
Genetic variability in biotransforming capability accounts for most of the large variation among humans. The Phase II acetylation reaction in particular is influenced by genetic differences in humans. Some persons are rapid and some are slow acetylators. The most serious drug-related toxicity occurs in the slow acetylators, often referred to as "slow metabolizers". With slow acetylators, acetylation is so slow that blood or tissue levels of certain drugs (or Phase I metabolites) exceeds their toxic threshold.
Examples of drugs that build up to toxic levels in slow metabolizers that have specific genetic-related defects in biotransforming enzymes are listed below:
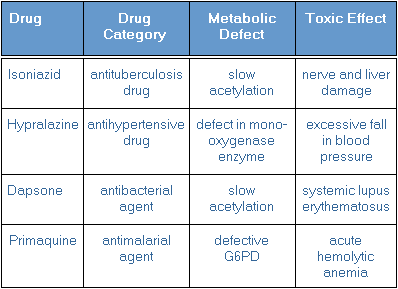
Poor nutrition can have a detrimental effect on biotransforming ability. This is related to inadequate levels of protein, vitamins, and essential metals. These deficiencies can decrease the ability to synthesize biotransforming enzymes. Many diseases can impair an individual's capacity to biotransform xenobiotics. A good example, is hepatitis (a liver disease), which is well known to reduce hepatic biotransformation to less than half normal capacity.
Enzyme inhibition and enzyme induction can be caused by prior or simultaneous exposure to xenobiotics. In some situations exposure to a substance will inhibit the biotransformation capacity for another chemical due to inhibition of specific enzymes. A major mechanism for the inhibition is competition between the two substances for the available oxidizing or conjugating enzymes, that is the presence of one substance uses up the enzyme that is needed to metabolize the second substance.
Enzyme induction is a situation where prior exposure to certain environmental chemicals and drugs results in an enhanced capability for biotransforming a xenobiotic. The prior exposures stimulate the body to increase the production of some enzymes. This increased level of enzyme activity results in increased biotransformation of a chemical subsequently absorbed. Examples of enzyme inducers are alcohol, isoniazid, polycyclic halogenated aromatic hydrocarbons (e.g., dioxin), phenobarbital, and cigarette smoke. The most commonly induced enzyme reactions involve the cytochrome P-450 enzymes.
Dose level can affect the nature of the biotransformation. In certain situations, the biotransformation may be quite different at high doses versus that seen at low dose levels. This contributes to the existence of a dose threshold for toxicity. The mechanism that causes this dose-related difference in biotransformation usually can be explained by the existence of different biotransformation pathways. At low doses, a xenobiotic may follow a biotransformation pathway that detoxifies the substance. However, if the amount of xenobiotic exceeds the specific enzyme capacity, the biotransformation pathway is "saturated". In that case, it is possible that the level of parent toxin builds up. In other cases, the xenobiotic may enter a different biotransformation pathway that may result in the production of a toxic metabolite.
An example of a dose-related difference in biotransformation occurs with acetaminophen (Tylenol®). At normal doses, approximately 96% of acetaminophen is biotransformed to non-toxic metabolites by sulfate and glucuronide conjugation. At the normal dose, about 4% of the acetaminophen is oxidized to a toxic metabolite; however, that toxic metabolite is conjugated with glutathione and excreted. With 7-10 times the recommended therapeutic level, the sulphate and glucuronide conjugation pathways become saturated and more of the toxic metabolite is formed. In addition, the glutathione in the liver may also be depleted so that the toxic metabolite is not detoxified and eliminated. It can react with liver proteins and cause fatal liver damage.

  
|
|
|
|

