|
 |
 |
 |
 |
|
|
|






Introduction

Distribution is the process whereby an absorbed chemical moves away from the site of absorption to other areas of the body. In this section we will answer the following questions:
 |
 |
 |
|  | How do chemicals move through the body?
|
 |
|  | Does distribution vary with the route of exposure?
|
 |
|  | Is a chemical distributed evenly to all organs or tissues?
|
 |
|  | How fast is a chemical distributed?
|
 |
|  | Why do some chemicals stay in the body for a long time whereas others are eliminated quickly?
|
 |
When a chemical is absorbed it passes through cell linings of the absorbing organ (skin, lung, or gastrointestinal tract) into the interstitial fluid (fluid surrounding cells) of that organ. Interstitial fluid represents about 15% of the total body weight. The other body fluids are the intracellular fluid (fluid inside cells), about 40% of the total body weight and blood plasma which accounts for about 8% of the body weight. However, the body fluids are not isolated but represent one large pool. The interstitial and intracellular fluids, in contrast to fast moving blood, remain in place with certain components (e.g., water and electrolytes) moving slowly into and out of cells. A chemical, while immersed in the interstitial fluid, is not mechanically transported as it is in blood.
A toxicant can leave the interstitial fluid by:
 |
 |
 |
|  | entering local tissue cells
|
 |
|  | entering blood capillaries and the blood circulatory system
|
 |
|  | entering the lymphatic system
|
 |
If the toxicant gains entrance into the blood plasma, it travels along with the blood, either in a bound or unbound form. Blood moves rapidly through the body via the cardiovascular circulatory system. In contrast, lymph moves slowly through the lymphatic system. The major distribution of an absorbed chemical is by blood with only minor distribution by lymph. Since virtually all tissues have a blood supply, all organs and tissues of the body are potentially exposed to the absorbed chemical.
Distribution of a chemical to body cells and tissues requires that the toxicant penetrate a series of cell membranes. It must first penetrate the cells of the capillaries (small blood vessels) and later the cells of the target organs. The factors previously described pertaining to passage across membranes apply to these other cell membranes as well. For example, concentration gradient, molecular weight, lipid solubility, and polarity are important, with the smaller, nonpolar toxicants, in high concentrations, most likely to gain entrance.
The distribution of a xenobiotic is greatly affected by whether it binds to plasma protein. Some toxicants may bind to these plasma proteins (especially albumin), which "removes" the toxicant from potential cell interaction. Within the circulating blood, the non-bound (free) portion is in equilibrium with the bound portion. However, only the free substance is available to pass through the capillary membranes. Thus, those substances that are extensively bound are limited in terms of equilibrium and distribution throughout the body. Protein-binding in the plasma greatly affects distribution, prolongs the half-life within the body, and affects the dose threshold for toxicity.
The plasma level of a xenobiotic is important since it generally reflects the concentration of the toxicant at the site of action. The passive diffusion of the toxicant into or out of these body fluids will be determined mainly by the toxicant's concentration gradient. The total volume of body fluids in which a toxicant is distributed is known as the apparent volume of distribution (VD ). The VD is expressed in liters.
If a toxicant is distributed only in the plasma fluid, a high VD results; however, if a toxicant is distributed in all sites (blood plasma, interstitial and intracellular fluids) there is greater dilution and a lower VD will result. Binding in effect reduces the concentration of "free" toxicants in the plasma or VD. The VD can be further affected by toxicants that undergo rapid storage, biotransformation, or elimination. Toxicologists determine the VD of a toxicant in order to know how extensively a toxicant is distributed in the body fluids. The volume of distribution can be calculated by the formula:
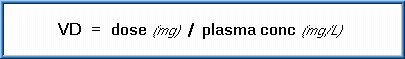
The volume of distribution may provide useful estimates as to how extensive the toxicant is distributed in the body. For example, a very high apparent VD may indicate that the toxicant has distributed to a particular tissue or storage area such as adipose tissue. In addition, the body burden for a toxicant can be estimated from knowledge of the VD by using the formula:

Once a chemical is in the blood stream it may be:
 |
 |
 |
|  | excreted
|
 |
|  | stored
|
 |
|  | biotransformed into different chemicals (metabolites)
|
 |
|  | its metabolites may be excreted or stored
|
 |
|  | the chemical or its metabolites may interact or bind with cellular components
|
 |
Most chemicals undergo some biotransformation. The degree with which various chemicals are biotransformed and the degree with which the parent chemical and its' metabolites are stored or excreted varies with the nature of the exposure (dose level, frequency and route of exposure).

  
|
|
|
|


